We are excited to announce the results of our latest clinical study, published in the December issue of the peer-reviewed Journal of Drugs in Dermatology.
In an open-label, two-week study in children and adults (ages 7 months to 57 years) with mild-to-moderate Atopic Dermatitus, significant improvement in eczema symptoms and Quality of Life for children and adults was observed after treatment with eczema cream with Gladskin’s patented endolysin ingredient.
Why Eczema Matters
Eczema, also called Atopic Dermatitis, is a chronic skin condition affecting 20% of children and 10% of adults in the U.S. It can cause constant, intense itching, redness, psychological stress, and sleep loss. In adults, this affects Quality of Life more than other chronic conditions such as heart disease or diabetes. In children, it has the second highest impact on QoL, following only cerebral palsy.
Current first-line treatments for eczema carry certain risks. According to the study:
“Topical corticosteroids (TCS) are first-line treatment for [eczema] flares since they effectively reduce inflammation. However, their use is limited to avoid developing skin atrophy in sensitive skin areas. Secondary infection, particularly by S. aureus, can be treated with broad spectrum or anti-staphylococcal antibiotics, but these can damage the beneficial skin microbiota and potentially lead to antibiotic resistance.”
A New Microbiome-Based Treatment Paradigm
Our skin has an active and diverse microbiome, made up of good and bad bacteria, just like our gut does. Up to 90% of people prone to eczema have a specific type of skin microbiome imbalance. When these bad bacteria outnumber the good bacteria it triggers eczema flare-ups. Non-prescription Gladskin Eczema Cream, with our patented endolysin, a microbiome balancing ingredient, restores a healthy balance to the skin microbiome, while moisturizing—and reduces eczema symptoms.
Significant Symptom Relief
The study published in the Journal of Drugs in Dermatology tracked patients between 7 months and 57 years of age with mild to moderate eczema who used a formulation with Gladskin’s patented endolysin ingredient at least once a day for two weeks. Standardized pictures were taken on days 0, 7, and 14.
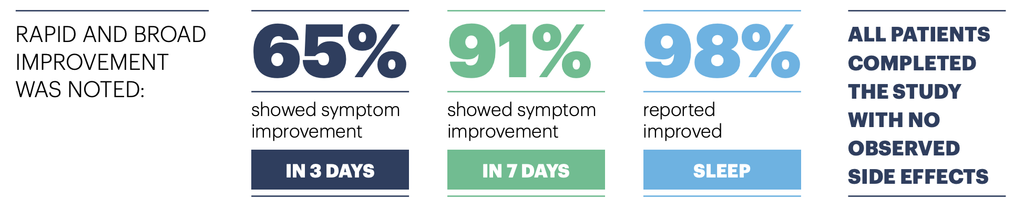
Results demonstrated reduced itch, redness, and tingling, while decreasing sleep loss, in virtually all subjects in this two-week study. Severity of symptoms and negative impact on quality of life rapidly and continuously declined.

Significant Improvement in the Quality of Life
100% of children and 95% of adults reported a higher QoL at the end of two weeks.

What This Means For 30 Million People
The authors of the study concluded that treatment with the ingredient in Gladskin produced a statistically and clinically significant reduction of eczema severity, while improving Quality of Life, and could be an alternative to traditional approaches for both adults and children. They note it has little risk of developing antibiotic resistance. In addition, they commented that there were no observed side effects in the study panel. They go on to state that the formulation with this Gladskin's patented endolysin may also be beneficial for preventing and reducing flares with ongoing use.
This research offers the 30 million adults and children in the U.S. who suffer from eczema, a new, safe and affordable way to reduce the impact of eczema on their lives.